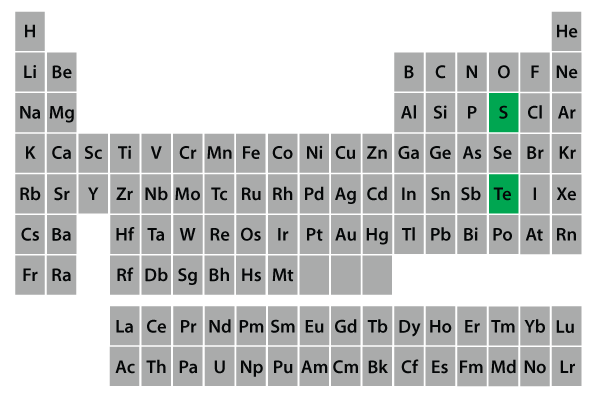
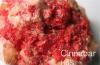
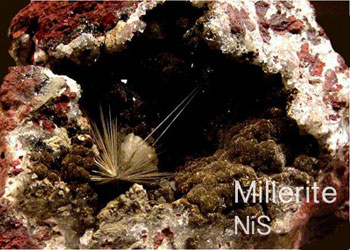
The minerals that make up the sulfide class are composed of metal cations (+2 charge) combined with sulfur. The sulfides form an important group of minerals which includes the majority of the ore minerals for iron, copper, nickel, lead, cobalt, zinc, and silver.
Most of the minerals in this class allow light to transmit through them (are opaque) with distinctive colors and colored streaks. The majority of these minerals in this class are also metallic, tend to have high densities, low hardness, exhibit electrical conductivity, and black or dark-colored streaks. They form in igneous environments.